ORLISTAT
by: Jiwani Peters
Orlistat is a novel dietary supplement that has been hot on the market as it is a unique anti-obesity (weight-loss) drug. It is known by two names on the market:
1. Alli – the over-the-counter version : 60 mg
2. Beacita – the over-the-counter version : 60 mg
3. Xenical – the doctor prescribed version : 120 mg *** the more potent version
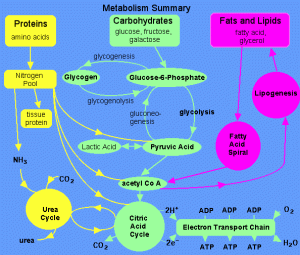
The manufacturers, Roche Pharmaceutical Company, claim a result of 30% decrease in fat absorption from exogenous fat sources i.e. the diet, hence weight loss. The key ingredient tetrahydrolipstatin is a derivative of lipostatin which inhibits gastic and pancreatic lipases. However because the Orlistat has the derivative; Tetrahydrolipstatin (THL), it selectively targets the action of pancreatic lipase and inhibits it. So it would interfere with dietary fat absorption, enhance weight loss and weight maintenance.
Tetrahydrolipstatin also has no adverse psycological effects such as addiction or dependence on it unlike other Amphetamines. Nor does this ingredient cause damage to the body (strokes, etc). This is because Tetrahydrolipstatin expresses itself externally in gastrointestinal tract only and Orlistat is lipophilic (water hating) so as such only less than 1% is absorbed into the blood stream which is 70% water i.e. mostly water.
Orlistat specifically targets and inhibits the action pancreatic lipase which is the main degradative lipase in lipid metabolism of long-chain fatty acids entering the digestive tract.
– Pancreatic Lipase
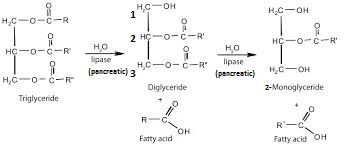
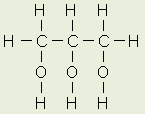
Pancreatic Lipase is made in the pancreas and secreted by it and goes into the small intestine, where it hydrolyses the ester bonds at Carbon 1 and Carbon 3 on the Triacylglyceride (TAG) molecule resulting in a mixture of 2-monoacylglycerol + fatty acids (which can be absorbed by the capillaries = weight gain)
Chemical equation: Triacylglycerol + 2 H2O 2-monoacylglycerol + 2 fatty acid anions
So by Orlistat selectively targeting and potently inhibiting this enzyme (90% action is stopped), it directly stops the hydrolysis and absorption of the fat by the body. Orlistat also inhibits gastric lipase, carboxylester lipase, and phospholipase A2 that helps in lipid digestion but advantageously has no effect on the activity trypsin, chymotrypsin, amylase (enzymes involved in protein digestion) and phospholipases.
- Orlistat’s Mechanism of Action
Orlistat binds covalently close to or at the active of pancreatic lipase. This new stable pancreatic lipase + inhibitor complex causes a conformational change which distorts the active site so it is exposed. Upon exposure, THL binds to the hydroxyl group on the Serine 152 residue at the active site of the lipase, as an ester bond. This obstructs the catalytic power of the lipase making it ineffective to do its hydrolyzing job.
So the dietary fats pass through the gastrointestinal tract without degradation and are excreted in the feces, so the feces is now of an oily nature; Steatorrhea.
- Overall EFFECTS
- Orlistat decreases lipids digestion and hence absorption. However Orlistat still needs to be used alongside a proper exercise regimen and a low-calorie diet. Because it is dependent on stopping absorption relative to the amount of fat ingested. Higher amounts of fat means that 30% of that amount will not be digested so the fat absorbed increase with increased fat intake.
- Orlistat improves insulin sensitivity, attributed to the reduction of free fatty acids (no obesity) so insulin clearance increases in activity and decreases hepatic glucose production. (Obesity is linked to diabetes). Studies have also shown that with long term Orlistat treatment, cholesterol levels and LDL cholesterol levels reduce, as fat from the diet would be present to be packaged into chylomicrons to be transported and deposited to the cells around the body.
- Orlistat has no drug-drug interaction with most of obesity drugs such as antihypertension, antidiabetics and cadiotonic medications. Nor does it have any interaction with lipid soluble vitamins or ß-carotene or serum hormones such as insulin, thyroxine and catecholamines. Orlistat seems to have no drug toxicity thus making it so revoluntary.
- Side effects:
– Include several mild gastrointestinal adverse effects: oily spotting, flatulence, and frequent loose stools.
– Vitamin D and β-carotene levels decreas (but remain within the normal range).
– The concentration of high-density lipoprotein (HDL) was also decreases.
References:
http://www.ncbi.nlm.nih.gov/pubmed/1899234
http://www.jnutbio.com/article/S0955-2863(98)00006-0/abstract